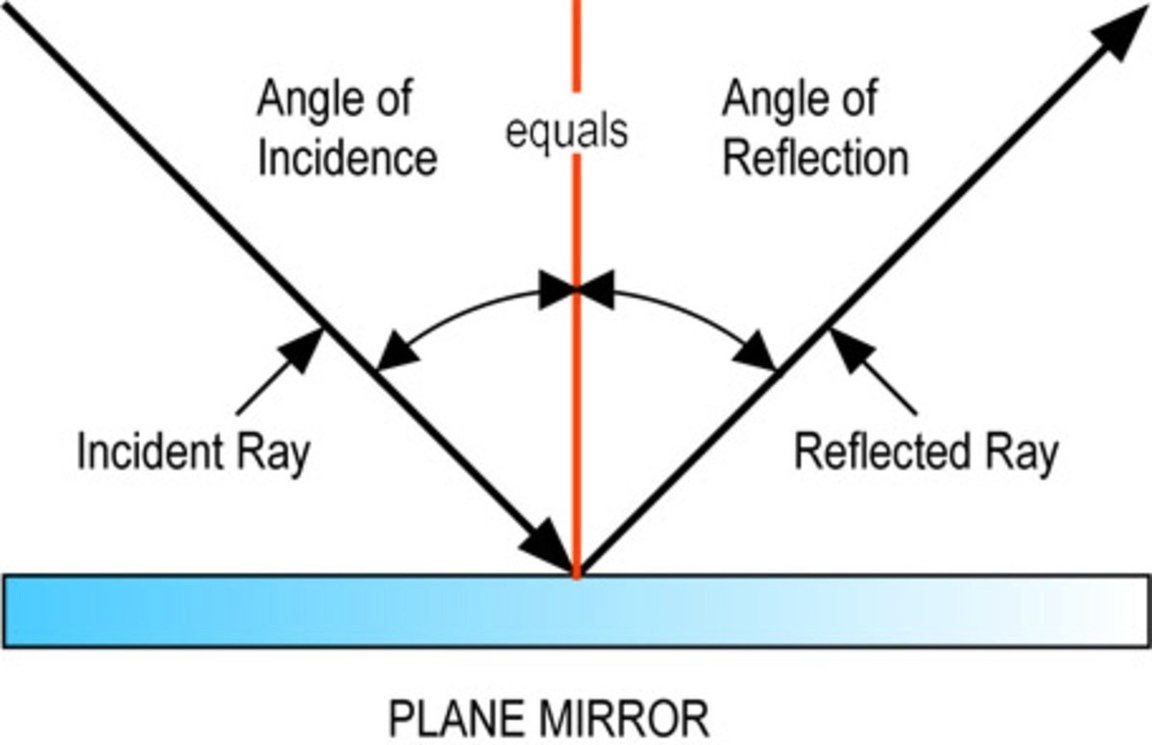
Qualitative Analysis Of Group I Ions: Qualitative analysis of Group I ions involves identifying and separating ions like sodium, potassium, and ammonium using specific chemical reactions and tests to observe their properties.
Qualitative Analysis Of Group I Ions